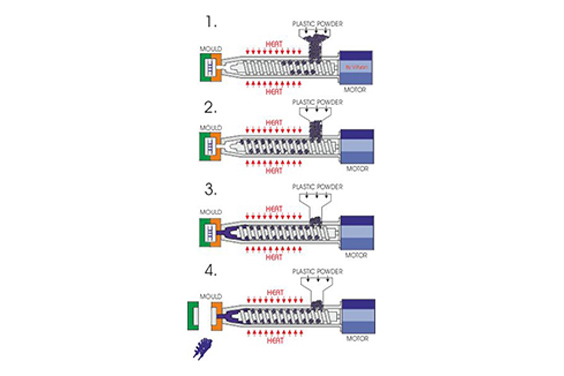
Choosing a medical device contract manufacturer is not a procurement task.
It is a decision about how manufacturing risk will be managed over the life of a regulated product.
This article explains how to evaluate contract manufacturers based on how medical manufacturing actually operates, rather than how it is commonly presented.
What a Medical Device Contract Manufacturer Actually Does
A medical device contract manufacturer does not simply execute production orders.
In practice, the manufacturer becomes part of the device’s manufacturing system.
Their processes, controls, and engineering decisions directly affect quality consistency, regulatory exposure, and delivery predictability.
Typical responsibilities include:
- Defining and controlling manufacturing processes
- Tooling development and process validation
- Medical-grade molding, assembly, and packaging
- Quality system execution and documentation
- Design transfer and ongoing change management
As a product moves from development into validation and commercial production, the manufacturer’s influence increases rather than decreases.
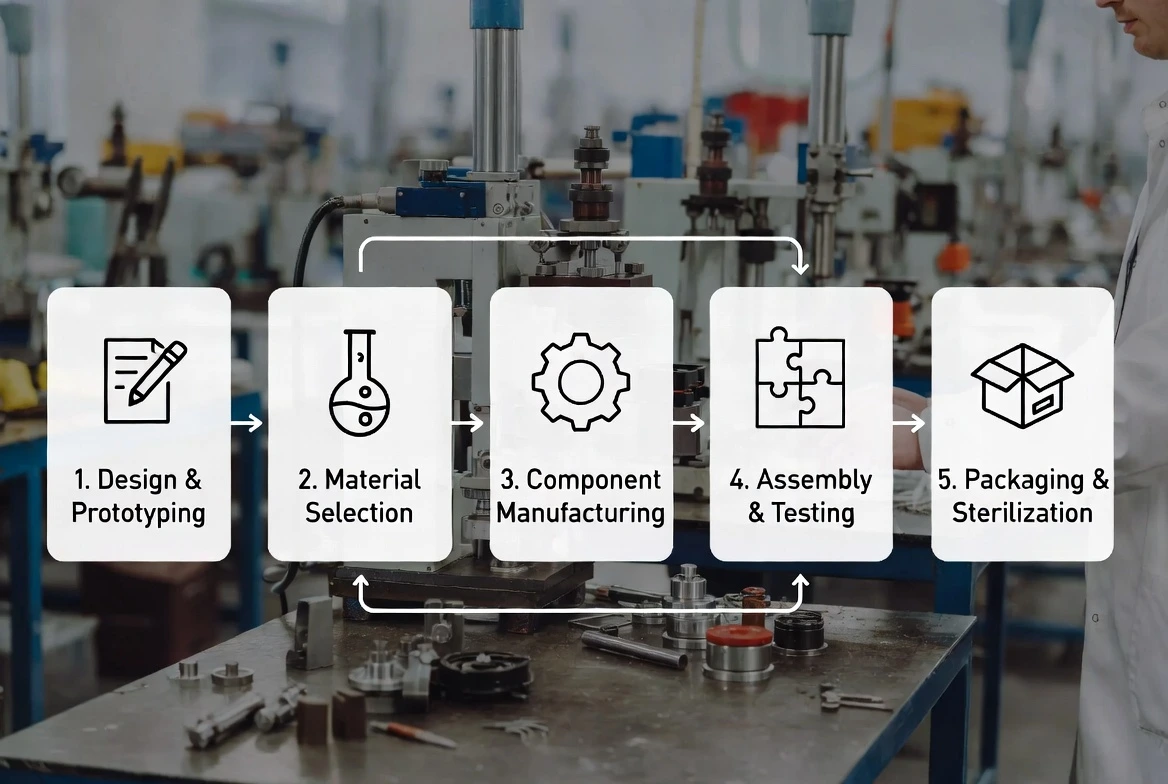
Why Manufacturer Selection Is a Risk-Control Decision
Medical device manufacturing operates under conditions where recovery options are limited.
Once tooling is validated, materials are locked, and documentation is submitted, changing manufacturers often requires:
- Tool transfer or revalidation
- Partial or full process requalification
- Documentation reconciliation across quality systems
These activities introduce schedule delays and regulatory exposure.
For this reason, selecting a contract manufacturer is fundamentally a risk-control decision.
The primary question is not whether a manufacturer can produce parts, but whether their systems make risk visible, manageable, and predictable.
Define Your Manufacturing Requirements First
Product Scope and Device Characteristics
Manufacturing evaluation should start with a clear definition of the device itself.
Key factors include:
- Device category and regulatory expectations
- Medical-grade material requirements and constraints
- Dimensional tolerance and repeatability needs
- Secondary processes such as insert molding or overmolding
For injection molded medical components, material behavior, tool design, and process window control often become the dominant risk drivers.
Production Volume and Lifecycle Expectations
Manufacturing needs change as products mature.
Early production may tolerate manual intervention.
Volume production does not.
Manufacturers should be evaluated on their ability to support:
- Engineering builds and validation runs
- Stable low-volume production
- Controlled scale-up to higher volumes
- Ongoing design or material changes without disruption
Lifecycle support is often more critical than initial capacity.
Evaluate Manufacturing Capabilities Beyond Equipment Lists
Process Capability and Technical Fit
Equipment lists provide limited insight into real manufacturing performance.
More reliable indicators include:
- Experience with similar medical device geometries
- Documented process controls and capability monitoring
- Engineering involvement during tooling and setup
In practice, manufacturers with prior exposure to comparable medical injection molding projects tend to identify tolerance, material flow, or tool wear risks earlier.

Scalability and Process Stability
Scale-up is where many manufacturing programs fail.
Common failure points include:
- Tool wear affecting dimensional stability
- Narrow process windows becoming unstable at higher throughput
- Increased scrap rates due to insufficient process monitoring
Manufacturers should demonstrate how they maintain process stability as volumes increase, not only how they achieve first-article approval.
Regulatory Compliance as an Operating System
Quality Management in Daily Operations
Compliance is not defined by certificates on a wall.
It is defined by how the quality system operates during normal production pressure.
A functional medical QMS governs:
- Document control and version discipline
- Material and batch traceability
- Nonconformance identification and closure
- Corrective and preventive action execution
When quality systems are poorly integrated into daily operations, issues are often detected late, after cost and schedule impact has already occurred.
Audit Readiness and Change Control
Medical manufacturing environments evolve continuously.
Tool updates, supplier changes, and process optimizations are inevitable.
Without structured change control, these updates quickly create documentation gaps.
Manufacturers that remain audit-ready typically maintain:
- Formal change evaluation procedures
- Clear approval paths for engineering and quality changes
- Consistent linkage between production changes and documentation
This discipline reduces disruption during inspections and customer audits.
Design Transfer and DFM Capability
Why Design Transfer Is a Common Failure Point
Design files rarely capture full manufacturing reality.
In practice, issues often appear when:
- Tolerances are technically correct but not manufacturable at scale
- Material selections behave differently in production tooling
- Assembly assumptions conflict with real process constraints
These gaps typically surface during tool trials or early validation builds.
Early Engineering Involvement
Manufacturers that engage engineering teams early reduce downstream rework.
Design for manufacturability helps:
- Improve yield and consistency
- Reduce unnecessary process complexity
- Control cost without sacrificing functional requirements
For precision medical injection molding, early DFM input often determines whether scale-up will be stable or fragile.
Supply Chain and Material Risk Management
Single-Source and Long-Lead Risks
Many medical-grade materials and components are single-source or long-lead.
When shortages occur, switching suppliers may require:
- Material equivalency studies
- Partial or full revalidation
- Regulatory notification or approval
Manufacturers should demonstrate awareness of these constraints during supplier selection and planning.
Supplier Control and Traceability
Effective supply chain control includes:
- Qualified supplier management
- Batch-level material traceability
- Incoming inspection aligned with device risk
These controls support both quality consistency and regulatory expectations.
Communication and Project Governance
Information Flow Between OEM and Manufacturer
Manufacturing failures often originate from documentation misalignment.
Common triggers include:
- Uncontrolled drawing revisions
- Assumptions made outside documented specifications
- Delayed communication of design changes
Structured information flow reduces these risks.
Ongoing Collaboration Models
As projects progress, governance needs change.
Effective manufacturers typically provide:
- Regular engineering and quality reviews
- Defined escalation paths for deviations
- Dedicated technical contacts during critical phases
Predictable communication is a core manufacturing control, not an administrative detail.
Cost Evaluation in a Regulated Environment
Why Lowest Price Often Increases Total Cost
Initial pricing rarely reflects total program cost.
Hidden costs frequently emerge from:
- Rework due to process instability
- Additional validation cycles
- Schedule delays caused by corrective actions
In regulated environments, these costs often exceed the difference between competing quotes.
Evaluating Cost Transparency
Transparent pricing clarifies:
- Tooling and setup assumptions
- Unit cost drivers
- Cost impact of design or process changes
This visibility supports better long-term planning.
Site Visits and Operational Transparency
Site visits remain one of the most reliable evaluation tools.
They reveal:
- How closely documented processes match reality
- Cleanroom discipline and material handling practices
- Workforce engagement and operational maturity
Routine operations often reflect true manufacturing culture more clearly than presentations.
When a One-Stop Manufacturing Model Makes Sense
Managing multiple suppliers increases coordination and traceability complexity.
An integrated manufacturing model is often appropriate when:
- Tooling, molding, assembly, and packaging are tightly coupled
- Documentation consistency is critical
- Volume transitions must remain controlled
In medical injection molding programs, consolidating design support, precision molding, cleanroom production, assembly, and packaging within a single quality system can reduce interface risk.
SeaSkyMedical operates as an ISO 13485-certified medical injection molding manufacturer, providing design support, tooling coordination, cleanroom molding, assembly, and packaging under a unified quality framework.
This approach is commonly applied where consistency and traceability are priority requirements rather than optional advantages.
Key Questions to Ask Before Final Selection
Before selecting a manufacturer, consider asking:
- How are process deviations detected during routine production
- What happens operationally when a nonconformance occurs
- How are design changes evaluated and approved
- How is supply continuity managed for critical materials
- How does the team support scale-up transitions
The structure and specificity of these answers often indicate operational maturity.
Making the Final Decision
No manufacturer eliminates risk entirely.
The goal is to select a partner whose systems surface risk early and manage it consistently.
Predictability, transparency, and process discipline are more reliable indicators than optimistic commitments.
Working With an Experienced Medical Injection Molding Partner
Medical injection molding requires control over material behavior, tooling precision, and process repeatability.
Manufacturers with established quality systems, cleanroom environments, and experience supporting regulated medical components are typically better positioned to manage these constraints across the product lifecycle.
SeaSkyMedical provides medical plastic injection molding services supported by ISO 13485 quality management systems, cleanroom manufacturing, and engineering-focused production support.
These capabilities are commonly applied to medical device components, consumables, and OEM molding programs.
Conclusion
Choosing a medical device contract manufacturer is a long-term decision with limited recovery options.
Manufacturers should be evaluated based on how their systems manage risk, maintain compliance, and support stable production over time.
Clear processes and disciplined execution remain the most reliable foundation for predictable outcomes.