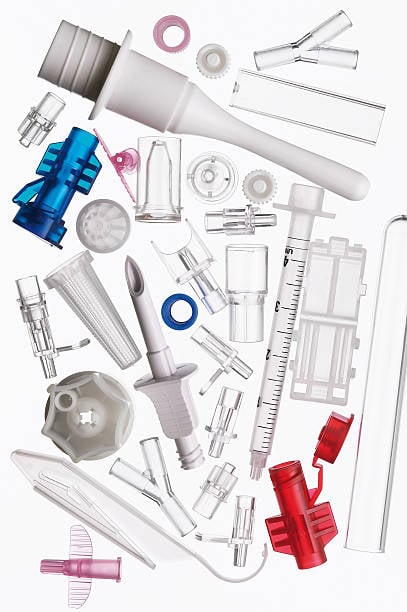
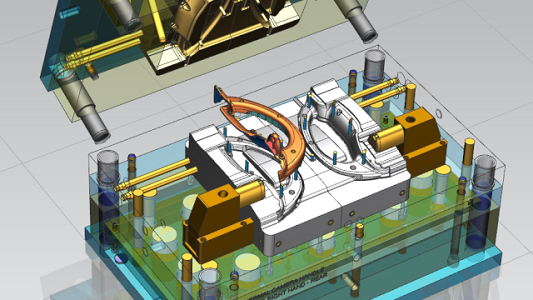
Contract manufacturing for medical devices refers to outsourcing defined manufacturing activities to a specialized supplier while the device owner retains design ownership and regulatory responsibility.
This model is commonly used when internal manufacturing capacity, process stability, or regulatory infrastructure is not yet established, and is widely applied in medical device contract manufacturing programs.
In medical injection molding, contract manufacturing is typically applied to processes that require validated tooling, controlled environments, and repeatable production conditions such as medical plastic injection molding.
How Contract Manufacturing Is Executed in Medical Injection Molding
IIn a medical molding context, contract manufacturing is not limited to part production.
It is a structured transfer of manufacturing execution under documented technical and quality requirements.
A typical execution flow includes:
- Design transfer with manufacturability and tolerance review
- Tooling design and mold fabrication with defined process parameters
- Pilot builds for process window verification
- Validation runs under defined parameters
- Volume production, inspection, and controlled packaging
Each stage is governed by process limits rather than design intent alone.alone.
Common Contract Manufacturing Models Used in Medical Devices
Component-Level Injection Molding
Only molded components are produced by the contract manufacturer.
Assembly and final packaging are handled elsewhere.
This model is suitable for:
- Medical device components molding with defined interfaces
- Tight-tolerance plastic parts requiring stable tooling
- Insert molding and overmolding features
Subassembly Manufacturing
The manufacturer produces molded parts and performs partial assembly.
This reduces internal handling steps and variability.
It is typically applied when multiple molded parts must maintain positional accuracy.
End-to-End Manufacturing Support
Manufacturing support extends from prototype tooling to cleanroom production and packaging.
This approach is commonly used for disposable medical products and consumables.
SeaSkyMedical typically supports this model through integrated product development programs that combine tooling development, process validation, and cleanroom manufacturing under a single execution framework.
Practical Benefits of Contract Manufacturing in Medical Device Manufacturing

Reduced Capital Exposure Under Defined Production Volumes
Medical injection molding requires upfront investment in tooling, equipment, and controlled environments.
Contract manufacturing avoids early capital lock-in when product volumes or lifecycle duration remain uncertain.
This benefit is most relevant when:
- Annual volumes are still being validated
- Multiple design iterations are expected
- Tooling amortization timelines are unclear
Access to Process-Specific Manufacturing Expertise
Injection molding performance is driven by process control rather than machine availability.
Contract manufacturers apply established controls for melt temperature, pressure profiles, and cooling behavior.
This is particularly relevant for:
- Micro injection molding medical applications
- Thin-wall components below typical thickness ranges
- Medical grade plastics with narrow processing windows
Predictable Transition From Design to Stable Production
Manufacturing risks often emerge during early production rather than during design.
Contract manufacturers commonly identify these risks through tooling reviews and pilot builds.
This allows manufacturers to move toward stable production without compressing validation steps.
Scalable Output Without Internal Line Disruption
Contract manufacturing supports both limited pilot runs and higher-volume production.
Capacity changes are absorbed within the manufacturer’s planning system rather than internal operations.
This approach is suitable when demand forecasts remain variable.
Reduced Internal Manufacturing Load
Outsourcing manufacturing execution reduces internal resource strain.
Internal teams remain responsible for design control and regulatory compliance.
Daily production management and equipment maintenance are handled externally.
Engineering Trade-Offs and Operational Constraints
Reduced Direct Visibility Into Daily Operations
Outsourcing limits real-time access to production activities.
This risk is commonly managed through defined reporting structures and routine audits.
Dependency on External Capacity Planning
Production schedules depend on the manufacturer’s capacity allocation.
Lead times must be confirmed during planning rather than assumed.
Alignment of Quality Systems
Quality alignment requires early coordination of documentation, inspection criteria, and change control.
Regulatory responsibility remains with the device owner.
Contract manufacturing does not eliminate compliance obligations.
When Contract Manufacturing Makes Sense in Medical Manufacturing
Contract manufacturing is commonly selected when one or more of the following conditions apply:
- Cleanroom injection molding is required
- Tooling investment must remain flexible
- Tolerances are tight and process stability is critical
- Initial volumes do not justify internal lines
It is less suitable when long-term volumes clearly justify dedicated facilities.ities.
Contract Manufacturing vs In-House Medical Injection Molding Decisions
| Decision Factor | Contract Manufacturing | In-House Manufacturing |
|---|---|---|
| Upfront capital | Lower | Higher |
| Process maturity | Established | Must be developed |
| Volume flexibility | High | Limited |
| Daily process control | Indirect | Direct |
| Cleanroom readiness | Available | Requires setup |
Medical Device Products Commonly Made Through Contract Manufacturing
Contract manufacturing is widely used for:
- Medical consumables injection molding
- Laboratory consumables molding
- Disposable medical device components
- Precision housings and fluid connectors
These products prioritize repeatability and process stability over rapid customization.
Manufacturing Capabilities That Directly Affect Production Outcomes

Manufacturing outcomes are influenced by environment control and process discipline.
Relevant capability factors include:
- Cleanroom medical manufacturing conditions
- Tooling accuracy and maintenance strategy
- Material traceability and handling procedures
- Inspection methods matched to tolerance requirements
SeaSkyMedical operates controlled injection molding processes with Class 7 and Class 8 cleanroom environments for medical plastic injection molding projects that require documented process stability.
Key Takeaways for Manufacturing Planning
Contract manufacturing is a widely used manufacturing model in the medical device industry.
Its effectiveness depends on technical alignment, realistic planning, and clearly defined responsibilities.
When applied under appropriate conditions, it allows manufacturers to balance cost exposure, flexibility, and production reliability across the product lifecycle.
For projects involving medical injection molding, tooling development, and cleanroom manufacturing, SeaSkyMedical can provide technical input during early manufacturing planning.
Contact SeaSkyMedical for more information.