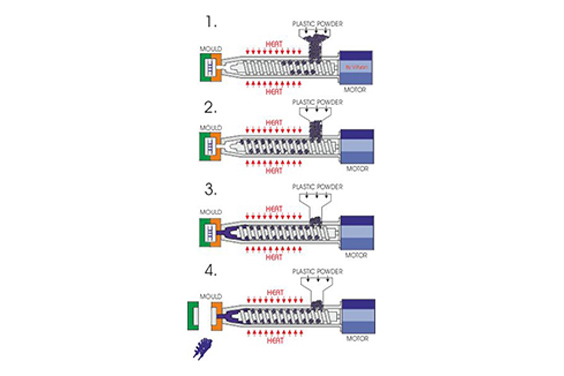
Injection molded medical components must provide exceptional performance while maintaining sterility and durability. Since patients and healthcare professionals interact with devices continuously, clear and intuitive interfaces are also critical for safety and efficacy. This is precisely where an advanced manufacturing process called in-mold decoration (IMD) comes into play.
IMD is an injection molding technology that embeds decorative or functional films directly into plastic components during the molding process. In the medical industry, IMD enhances the aesthetics of plastic parts and improves device performance, usability, and brand recognition. Now, let’s take a closer look at IMD.
What is IMD (in-mold decoration) Injection Molding?
IMD is a process in which a pre-printed or pre-finished film (often called a foil, inlay, or skin) is placed inside the mold cavity before plastic resin is injected. The molten plastic resin then flows behind the film and bonds with it permanently as the part cools and solidifies. The result is a single, inseparable component in which the decoration is protected by the plastic surface.
This process is fundamentally different from traditional post-molding decoration methods, such as painting, pad printing, or applying adhesive labels. These methods add layers on top of the finished part, which can chip, scratch, wear off, or create edges where contaminants can hide—a significant concern in medical environments.
Key Characteristics of IMD Films for Medical
Multi-Layer Construction
They often include a printable top layer, a protective clear coat, and a bonding layer.
Medical-grade materials
Films are compatible with medical-grade plastics, such as PC, ABS, and PP, and can be formulated for biocompatibility.
Sterilization resistance
High-quality IMD films are engineered to withstand common sterilization methods, such as gamma radiation, ethylene oxide (EtO), and autoclaving, without delaminating, yellowing, or losing adhesion.
How Does the IMD Process Work?
The IMD process is precise and repeatable, making it ideal for high-volume medical device production. It typically involves four key stages:
Film Creation & Printing
First, a graphic film (usually polyester or polycarbonate) is printed with the required designs. This can include:
Permanent Branding: Logos, product names, and regulatory marks (CE, FDA).
User Interfaces: Keypad graphics, symbols, icons, scales, and instructional text.
Aesthetic Finishes: Wood grains, metallic looks, or specific brand colors.
Advanced printing techniques allow for complex, high-resolution graphics that are impossible to achieve with traditional in-mold labeling.
Film Forming (Optional)
For parts that are not flat, the printed film may need to be thermoformed into a specific 3D shape that matches the contours of the final mold cavity. This step ensures the film fits perfectly without wrinkling.
In-Mold Placement & Molding
This is the core of the process. The formed film is robotically placed and precisely secured in the injection mold. The mold closes, and molten plastic is injected at high pressure behind the film. The heat and pressure activate the film’s bonding layer, creating a permanent molecular bond with the substrate plastic.
Part Ejection & Finishing
After cooling, the mold opens, and a fully finished, decorated part is ejected. The part is complete out of the mold, requiring no secondary decoration steps. Any excess film (carrier web) around the edges is trimmed away, leaving a clean, seamless part.
Key Applications of IMD in Medical Injection Molding
IMD offers numerous benefits, including durability, cleanliness, and design integration, making it a perfect fit for a variety of medical applications.
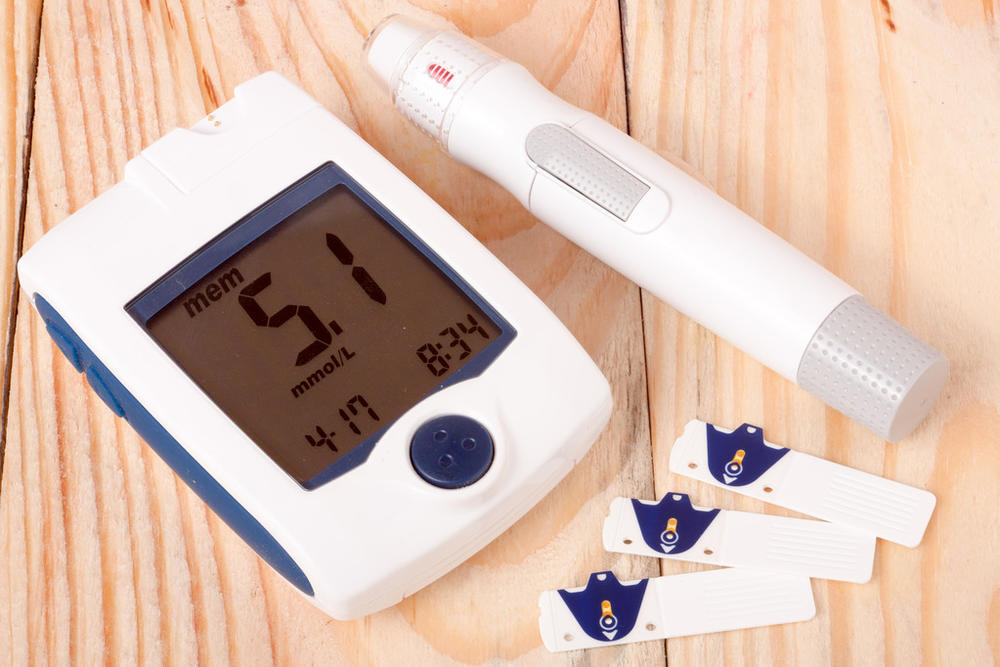
Diagnostic and monitoring equipment
Housings and front panels: IMD creates scuff- and chemical-resistant surfaces for blood analyzers, glucose meters, and patient monitors. Complex control panel graphics are permanently sealed under a clear plastic layer.
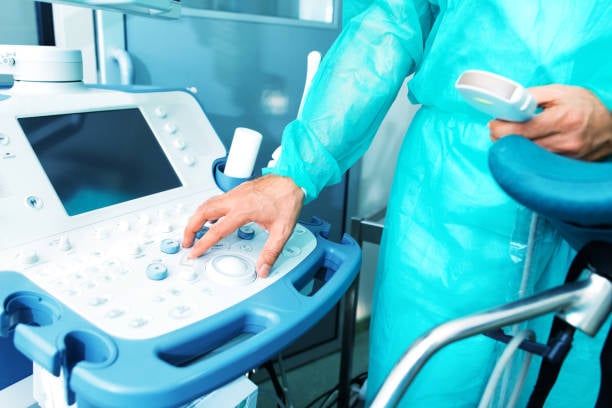
Branding and compliance: Serial numbers, model information, and required certification marks can be molded directly into the device to ensure that they never wear off over the product’s lifecycle.
Surgical and Handheld Instruments
Ergonomic Handles: Non-slip texture zones and functional grip patterns can be integrated via the film.
Scale and measurement markings: Precise measurement scales are embedded and protected on surgical tools and adjustable devices, remaining legible after hundreds of autoclave cycles.
Drug Delivery Systems
Inhalers and injectors: Dose counters, usage instructions, and brand colors are integral to the device body and are resistant to oils from skin contact and cleaning agents.
Intuitive Design: Color-coded buttons or sections (e.g., for different dosage settings) are molded in place to reduce user error.
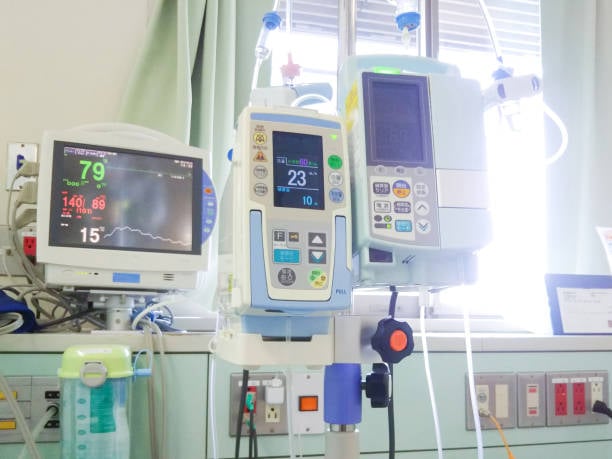
Durable Medical Equipment and Consumables
Control Panels: IMD panels offer a smooth, wipe-clean surface without gaps for fluids to penetrate for infusion pumps, hospital bed controls, and dialysis machines.
Brand Differentiation: In a competitive market, IMD enables high-quality, aesthetically pleasing finishes on devices such as nebulizers and portable oxygen concentrators, thereby improving patient perception.
Why Choose IMD for Your Next Medical Device Project?
Enhanced Durability & Sterilizability
The decoration is encapsulated, making it resistant to abrasion, chemicals, and the rigors of repeated medical sterilization.
Superior Cleanability
The seamless, non-porous surface has no edges or gaps for biological contaminants to lodge in, which supports infection control protocols.
Cost-Effectiveness
Consolidating molding and decoration into one automated step eliminates secondary processes (painting, printing, labeling), reducing labor, inventory, and potential error.
Design Freedom & Aesthetics
It enables complex, multicolored graphics and special effects that are difficult or impossible with other methods while maintaining an integrated, high-end look.
Conclusion
IMD is more than a manufacturing process; it’s a design enabler for the medical industry. By fusing functionality with permanent, high-quality aesthetics, IMD helps create medical devices that are safer, cleaner, more durable, more intuitive, and more trusted by the end user. When developing your next medical plastic component, consider IMD to elevate its performance and human-centric design.
Is IMD the right solution for your medical device? Contact Seasky’s engineering team today to discuss how this innovative technology can be integrated into your product design to achieve superior results.