If you are designing a product that touches the human body, choosing the right plastic is critical. Two common terms you will hear are “food grade” and “medical grade.” While they might sound similar, they represent vastly different levels of safety and testing. Think of it this way: All medical grade plastics meet food grade requirements, but food grade plastics do not meet medical grade standards.
Using the wrong material can lead to product failure, regulatory rejection, and most importantly, risk to patient health. This guide will break down the key differences in simple terms.
Core Difference: The Goal and The Risk
The main difference lies in the goal of the material and the level of risk involved.
Food Grade Plastic has one primary job: Chemical Safety.
Its purpose is to prevent harmful chemicals from the plastic (like additives or residues) from migrating into food or drink under normal conditions. The concern is long term, low level chemical exposure from ingestion. Think of a water bottle or a food container.
Medical Grade Plastic has a much higher and broader job: Biological Safety and Performance.
Its purpose is to be biocompatible. This means it must not cause any harmful reaction when it touches the human body. Contact can be with skin, mucous membranes, blood, or even inside the body as an implant. The risks are immediate and serious, including toxicity, inflammation, infection, or blood clotting. Think of a syringe, a surgical tool, or a pacemaker component.

The Standards and Rules: A Different Rulebook
This is where the biggest practical difference lies. The rules for medical plastics are much stricter.
For Food Grade Plastic:
- The Rulebook: It follows regulations for Food Contact Materials. Examples are the FDA’s CFR Title 21 in the USA or EU Regulation 10/2011 in Europe.
- The Main Test: Migration Testing. Scientists test how much of certain chemicals can move from the plastic into food simulants (like liquids that act like food) under set conditions like heat and time. If migration is below the safe limit, the plastic passes.
- Certification: The plastic resin supplier provides a document stating their material complies with the relevant food contact regulation.
For Medical Grade Plastic:
- The Foundation: It must be produced under a Quality Management System certified to ISO 13485. This is a special system for medical devices that controls every single step, ensuring consistency and traceability.
- The Core Hurdle: Biological Evaluation (ISO 10993). This is not just one test. It is a series of tests based on how the device will contact the body. Tests can include:
- Cytotoxicity: Does the plastic kill cells?
- Sensitization: Can it cause an allergic reaction?
- Irritation: Does it irritate skin or tissue?
- Systemic Toxicity: Does it cause harmful effects elsewhere in the body?
- The Real World Test: Medical grade plastic must also survive sterilization (like high heat, radiation, or gas) without breaking down or becoming toxic.
- Traceability: Every batch of medical grade resin and the parts made from it must be fully traceable from start to finish.
Material Properties: Purity, Consistency, and Traceability
The raw plastic itself is held to a higher standard for medical use.
| Property | Food Grade Plastic | Medical Grade Plastic |
| Purity & Consistency | High purity, low risk of chemical migration. | Extremely high purity. Tighter limits on impurities and additives. Performance must be identical from batch to batch. |
| Additives | Uses approved additives for color or flexibility. | Additive use is highly restricted. Every colorant, stabilizer, or plasticizer must be proven biocompatible. |
| Traceability | Batch tracking is common. | Full chain traceability is mandatory. We must know the full history of the material. |
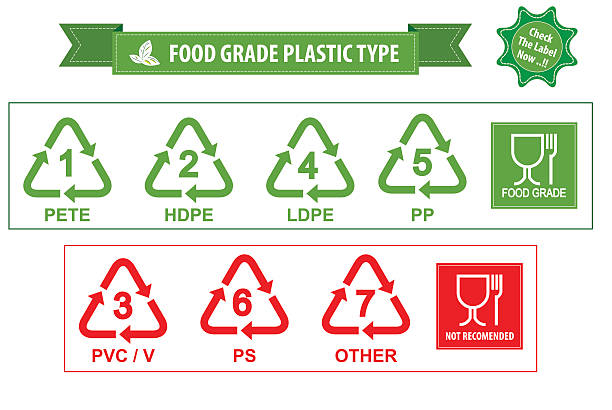
Where Are They Used? Application Examples
Food Grade Plastic is used for:
- Water and soda bottles
- Food storage containers and packaging
- Kitchen utensils and disposable cutlery
- Components in coffee makers or water filters
Medical Grade Plastic is used for:
- Critical Devices: Surgical instruments, implantable components (joints, meshes), heart valves.
- Blood Contact Devices: Syringes, IV tubes, blood bags, dialysis filters.
- Diagnostic Tools: Test cartridges, pipette tips, sample vials.
- Drug Delivery: Inhalers, insulin pens, pre filled syringe barrels.
A Special Note on Drug Packaging: Packaging for pills or liquid medicine often needs standards beyond basic food grade (like USP Class VI testing), placing it closer to medical grade requirements due to the direct interaction with pharmaceuticals.
Conclusion: Choosing the Right Path for Your Product
The choice between food grade and medical grade plastic is not optional—it is dictated by your product’s intended use and the regulations that govern it.
For a consumer product that holds food or drink, a certified food grade plastic is the correct and cost effective choice.
For any device that is classified as a medical device (for diagnosis, treatment, or monitoring of disease), you must use medical grade materials and manufacture in an ISO 13485 certified facility. There is no substitute.
Using food grade plastic in a medical device is a serious risk. It may lack the necessary biocompatibility, fail during sterilization, or lead to regulatory failure because it cannot meet the required documentation and traceability standards.
At our medical injection molding facility, we work exclusively with certified medical grade polymers and operate under the ISO 13485 quality system. We understand these critical differences and ensure every component we mold meets the stringent requirements for safety, traceability, and performance that the medical industry demands.
Need guidance on material selection for your medical device? Contact our engineering team. We can help you navigate the specifications and choose the right, compliant material for your application.